| Subject |
has pronunciation |
has thermal neutron capture cross section |
has rigidity modulu |
has synonym |
has isotope mass range |
has heat of vaporization |
has discoverer |
has thermal conductivity |
has discovery date |
has occurrence |
has name origin |
has linear expansion coefficient |
has heat of fusion |
has specimen |
has main mining area |
has critical pressure |
has daily dietary intake |
has lethal intake |
has ocean residence time |
has mineral |
has melting point |
has neutron scattering length |
has Curie temperature |
has image |
has covalent radii |
has symbol name origin |
has term symbol |
has crystal cell dimension |
has poisson's ratio |
has mass of element in person |
has reserve |
has ocean oxidation state |
has young's modulu |
react |
has state |
has heat capacity |
has bulk modulu |
has van der Waals radii |
has molar volume |
has definition |
has boiling point |
has hazard |
has biological role |
has ionic radii |
has electrical resistivity |
has relative atomic mass |
has chief source |
has electron affinity |
has registry number |
has density |
has atomic radii |
has atomic number |
has mass magnetic susceptibility |
has hardness |
has discovery location |
has number of isotope |
has toxic intake |
has abundance |
has atomic emission line |
reacts with |
has longest lived isotope |
has filling orbital |
has symbol |
has use |
has world production |
has electron configuration |
has number of proton |
has mass absorption coefficient |
has level in human |
has electronegativity |
has critical temperature |
has appearance |
has group |
|---|
| arsenic | ahrs-nik | 4.30 barns | | pnictogen | 67 to 87 | 31.9 kJ mol-1 | Albertus Magnus | 50.0 W m-1 K-1 at 300 K for α form | 1250 | ocean | arsenikon = yellow orpiment from Greek | 4.6 × 10-6 K-1 | 27.7 kJ mol-1 | available as powder. Danger ! | not much mined as such because more than required is produced as a by-product of refining certain sulfide ores | | 0.04 to 1.4 mg | 50 to 300 mg | 90000 years | arsenopyrite, conichalcite, enargite, lollingite, olivenite, orpiment and realgar | 1090 K for α form under pressure | 0.658 × 10-12 cm | |  | 121 pm | | 4S3/2 in ground state | c = 10.548 pm for β form | | 0.5 to 15 mg for a 70 kg average person depending on diet | | V | | | | 20.786 J K-1 mol-1 for gas at constant pressure of 0.1 MPa at 298.15 K | | 200 pm | 15.9 cm3 for β form | a metalloid element with two main forms, grey α arsenic and β arsenic | 889 K sublimes | salts and arsine gases are very poisonous. Stimulates metabolism in small doses, but it is carcinogenic and possibly teratogenic | essential to some species including humans | 69 pm for As3+ | 26 × 10-8 Ω m at 273 K | 74.92159 in units of 12C = 12.000 | arsenopyrite, realgar and orpiment | 78 kJ mol-1 from As to As- | 7440-38-2 for Chemical Abstracts System database | 4700 kg m-3 for solid at 293 K (β form) | 125 pm | 33 | -3.97 × 10-9 kg-1 m3 for β form | brittle for α form | Germany | 22 | 5 to 50 mg | 1.75 × 10-3 p.p.m. in deep Pacific seawater | 454.348 nm for As II | hot acids and molten NaOH, tarnishes burns in oxygen | | | As | alloys, semiconductors, pesticides, wood preservatives and glass | 47000 tonnes per year for As2O3 | [Ar]3d104s24p3 in ground state | 33 | 69.7 cm2 g-1 for MoKα X-ray diffraction | 0.009 to 0.65 p.p.m. in muscle | 2.18 Pauling | | metallic for α form | 15 |
| barium | | | | atom | | | | | | ocean | | | | | | | | | 10000 years | | | | |  | | | | | | | | II | | | | | | | | | | | | | | | | | | | | | | malleable, extrudable and machinable | | | | | | | | | | | | | | | | | | | |
| beryllium | be-ril-iuhm | 0.0092 barns | 156 GPa | atom | 6 to 11 | 308.8 kJ mol-1 | Nicholas Louis Vauquelin | 200 W m-1 K-1 at 300 K | 1797 | ocean | beryllos = beryl from Greek | 11.5 × 10-6 K-1 | 9.80 kJ mol-1 | lumps, powder. Danger ! | Brazil, USA, Madagascar, Germany, Czech Republic, Russia, India | | 0.01 mg | 317 mg kg-1 acetate in rat | 4000 years | beryl, bertrandite, chrysoberyl, gadolinite, herderite | 1551 ± 5 K | 0.779 × 10-12 cm | |  | 89 pm | | 1S0 in ground state | a = 255.15 pm for β-Be | 0.02 GPa | 0.036 mg for a 70 kg average person | 400000 tonnes | II | 318 GPa | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 110 GPa | | 4.88 cm3 | Silvery-white, lustrous, relatively soft metal which is unaffected by air or water, even at red heat. Rare and fragile element. Nuclear reactions in stars destroy it. Most and possibly all beryllium originated when cosmic rays smashed into heavier atoms in space and split them into lighter ones, such as beryllium. | 3243 K under pressure | inhalation of dust causes severe and irreparable lung damage | none | 34 pm for Be+ | 4.0 × 10-8 Ω m at 293 K | 9.012182 in units of 12C = 12.000 | beryl, bertrandite | -18 kJ mol-1 from Be to Be- | 7440-41-7 for Chemical Abstracts System database | 1847.7 kg m-3 for solid at 293 K | 113 pm | 4 | -1.3 × 10-8 kg-1 m3 for solid | malleable, extrudable and machinable | Paris, France | 6 including nuclear isomers | 13 mg kg-1 in rat | 22 × 10-8 p.p.m. in deep Pacific seawater | 527.081 nm for Be II | | beryllium 9 | | Be | copper alloys used to make spark proof tools | 364 tonnes year-1 | [He]2s2 in ground state | 4 | cm2 g-1 0.298 for MoKalpha X-ray diffraction | 0.00075 p.p.m. in muscle | 1.57 Pauling | | | |
| cadmium | | | | atom | | | | | | in sulfide minerals | | | | | | | | | 30 years | | | | |  | | | | | | | | II | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
| calcium | kal-sium | 0.43 barns | 7.9 GPa | atom | 36 to 51 | 149.95 kJ mol-1 | Sir Humphry Davy | 200 W m-1 K-1 at 300 K | 1808 (isolated) | in silicate materials such as igneous rocks | calx = lime from Latin | 22 × 10-6 K-1 | 9.33 kJ mol-1 | granules, pieces or turings. Care ! | common everywhere | | 600 - 1400 mg | 6.45 grams kg-1 in carbonate form for rat | 1 × 106 years | anhydrite, aragonite, calcite, dolomite, gypsum, shortite, vaterite | 1112 K | 0.476 × 10-12 cm | | 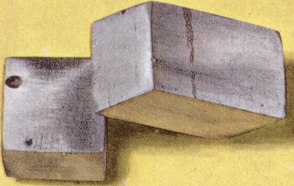 | 174 pm | | 1S0 in ground state | a = pm | 0.31 GPa | 1.00 kg for a 70 kg average person | almost unlimited | II | 19.6 GPa | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 17.2 GPa | | 25.86 cm3 | silvery-white, relatively soft metal | 1757 K | calcium compounds are only toxic via the other elements they contain | essential to all species | 106 pm for Ca2+ | 3.43 × 10-8 Ω m at 293 K | 40.078 in units of 12C = 12.000 | calcite, dolomite, gypsum (used in cement and plaster) anhydrite (used to make H2SO4) | -186 kJ mol-1 from Ca to Ca- | 7440-70-2 for Chemical Abstracts System database | 1365 kg m-3 for liquid at 1112 K melting point | 197 pm for α-form | 20 | +1.4 × 10-8 kg-1 m3 for solid | malleable, extrudable and machinable | London, England | 16 including nuclear isomers | non-toxic | 440 p.p.m. in deep Pacific seawater | 422.673 nm for Ca I (used in atom absorption spectrometry) | oxygen and water but soon forms a thin protective oxide-nitride film preventing further corrosion | | | Ca | CaO used in metallurgy, water treatment, chemicals industry, cement, etc | 112 × 106tonnes year-1 for lime, CaO | [Ar]4s2 in ground state | 20 | 18.3 cm2 g-1 for MoKα X-ray diffraction | 140 - 700 p.p.m. in muscle | 1.00 Pauling | | | |
| carbon | kar-bon | 0.0035 barns | | atom | 9 to 16 | 710.9 kJ mol-1 | | 990 - 2320 W m-1 K-1 for diamond at 298 K | pre-historic | in planet metallic core and associated with iron | carbo = charcoal from Latin | 1.19 × 10-6 K-1 for diamond | 105.1 kJ mol-1 | amorphous, fullerenes, bucky tubes, diamond, graphite and soot. Safe. | diamond deposits in South Africa, USA, Russia, Brazil, Zaire, Sierra Leone, Ghana, Canada | | 300 g | | 800000 years | diamond, graphite, calcium magnesium carbonates, fossil fuel | 800 K for buckminsterfullerene (sublimes) | 0.66460 × 10-12 cm | |  | 60 pm for triple bonds | | 3P0 in ground state | a = 1414 pm for buckminsterfullerene | | 16 kg for a 70 kg average person | large for tar sands | IV | | | | 20.838 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 185 pm | 3.42 cm3 for diamond | pure forms occur as graphite, diamond and buckminsterfullerene C60 | 5100 K (sublimes) | carbon black is a nuissance but not dangerous, although soot may harbour carcinogenic materials | DNA constituent, organic molecules required for life | 260 pm for C4- | 1 × 1014 Ω m for buckminsterfullerene at 293 K | 12.011 in units of 12C = 12.000 | graphite | 121.9 kJ mol-1 from C to C- | 7440-44-0 for Chemical Abstracts System database | 1650 kg m-3 for buckminsterfullerene at 293 K | 77 pm | 6 | -6.2 × 10-9 kg-1 m3 for diamond | | | 8 including nuclear isomers | non-toxic, but some compounds can be very toxic such as CO or CN- | 28 p.p.m. in deep Pacific seawater | 723.642 nm for C II (strong) | almost everything | carbon 12 | | C | coke in steel, carbon black in printing, as a filler, activated charcoal for water treatement and respirators | 8.6 × | [He]2s22p2 in ground state | 6 | 0.625 cm2 g-1 for MoKα X-ray diffraction | 670000 p.p.m. in muscle | 2.55 Pauling | | | 14 |
| chromium | kroh-mi-uhm | 3.1 barns | 115.3 GPa | atom | 45 to 57 | 348.78 kJ mol-1 | Nicholas Louis Vauquelin | 93.7 W m-1 K-1 at 300 K | 1780 | in sulfide minerals | chroma = colour from Greek | 6.2 × 10-6 K-1 | 15.3 kJ mol-1 | chips, chunks, crystallites or powder. Safe. | Turkey, South Africa, Zimbabwe, Russia, Philipipines | | 0.01 - 1.2 mg | 70 mg kg-1 metal taken oraly in humans | 10000 years | chromite, crocoite | 2130 ± 20 K | 0.3635 × 10-12 cm | |  | | | 7S3 in ground state | a = 288.46 pm | 0.21 GPa | 14 mg for a 70 kg average person | 1 × 109 tonnes | VI | 279 GPa | | | 20.79 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 160.2 GPa | | 7.23 cm3 | hard, blue-white metal which resists oxidation in air, can be polished to a high shine | 2945 K | poison by ingestion, suspected carcinogen, chromates are corrosive to tissue | essential to some species, including humans, stimulates metabolism | 84 pm for Cr2+ | 12.7 × 10-8 Ω m at 293 K | 51.9961 in units of 12C = 12.000 | chromite | 64.3 kJ mol-1 from Cr to Cr- | 7440-47-3 for Chemical Abstracts System database | 6460 kg m-3 for liquid at 2130 K melting point | 125 pm | 24 | +4.45 × 10-8 kg-1 m3 for solid | | Paris, France | 13 including nuclear isomers | 200 mg | 2.5 × 10-4 p.p.m. in deep Pacific seawater | 520.844 nm for Cr I | HCL and H2SO4 by disolving | chromium 52 | | Cr | alloys, plating and metal ceramics | 9.6 × 106 tonnes year-1 for chromite ore | [Ar]3d54s1 in ground state | 24 | 31.1 cm2 g-1 for MoKalpha X-ray diffraction | 0.024 - 0.84 p.p.m. in muscle | 1.66 Pauling | | | a column number in the table of the elements |
| copper | | | | atom | | | | | | ocean | | | | | | | | | 3000 years | | | | |  | | | | | | | | II | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
| dysprosium | | | | lanthanide | | | | | | ocean | | | | | | | | | 300 years | | | | | 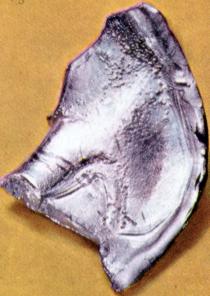 | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| erbium | | | | lanthanide | | | | | | ocean | | | | | | | | | 400 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| europium | | | | lanthanide | | | | | | ocean | | | | | | | | | 500 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| gadolinium | | | | lanthanide | | | | | | ocean | | | | | | | | | 300 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| germanium | | | | atom | | | | | | in planet metallic core and associated with iron | | | | | | | | | 20000 years | | | | |  | | | | | | | | IV | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 14 |
| holmium | | | | lanthanide | | | | | | ocean | | | | | | | | | 103 to 105 years | | | | | 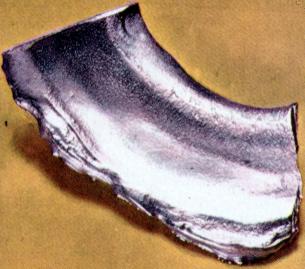 | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| iodine | | | | group VII element | | | | | | atmosphere of a planet or asteroid | | | | | | | | | 300000 years | | | | | 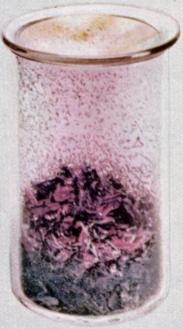 | | | | | | | | V | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 17 |
| iron | iy-on ? | 2.56 barns | 81 GPa for steel | atom | 49 to 63 | 351.0 kJ mol-1 | | 80.2 W m-1 K-1 at 300 K | 2500 B.C. | in planet metallic core and associated with iron | iron from Anglo-Saxon | 12.3 × 10-6 K-1 | 14.9 kJ mol-1 | chips, filings, foil, granules, powder and wire. Safe. | USA, Canada, Sweden, South Africa, Russia, India, Japan | | 6 - 40 mg | 7 - 35 grams | 98 years | goethite, hematite, lepidocrocite, magnetite, siderite, ... | 1808 K | 0.954 × 10-12 cm | |  | 116 pm | ferrum = iron from Latin | 5D4 in ground state | a = 293.22 pm for δ-Fe | 0.27 GPa for steel | 4.2 kg for a 70 kg average person | 1.1 × 1011 tonnes | III | 208 GPa for steel | | | 25.677 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 160 GPa for steel | | 7.09 cm3 | lustrous, silvery and soft or workable metal when absolutely pure | 3023 K | deficiency leads to anemia but excess causes liver and kidney damage | essential to all species | 82 pm for Fe3+ | 9.71 × 10-8 Ω m at 293 K | 55.845 in units of 12C = 12.000 | hematite, magnetite, goethite, lepidocrocite, siderite | 15.7 kJ mol-1 from Fe to Fe- | 7439-89-6 for Chemical Abstracts System database | 7035 kg m-3 for liquid at 1808 K melting point | 124 pm | 26 | ferromagnetic | | | 16 including nuclear isomers | 200 mg Iron (II) compounds are more toxic than iron (III) | 1 × 10-4 p.p.m. in deep Pacific seawater | 385.991 nm for Fe I (strong) | dilute acids by disolving | iron 56 | | Fe | steel etc... | 7.16 × 108 tonnes year-1 | [Ar]3d64s2 in ground state | 26 | 38.5 cm2 g-1 for MoKα X-ray diffraction | 180 p.p.m. in muscle | 1.83 Pauling | | | a column number in the table of the elements |
| lanthanum | | | | lanthanide | | | | | | ocean | | | | | | | | | 200 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| lutetium | | | | lanthanide | | | | | | ocean | | | | | | | | | 4000 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| neodymium | | | | lanthanide | | | | | | ocean | | | | | | | | | 500 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| nickel | nik-el | 4.49 barns | 76.0 GPa | atom | 53 to 67 | 371.8 kJ mol-1 | A.F. Cronstedt | 90.7 W m-1 K-1 at 300 K | 1751 | in planet metallic core and associated with iron | kupfernickel = Devil's copper or St Nicholas's copper from German | 13.3 × 10-6 K-1 | 17.6 kJ mol-1 | foil, powder, rod, slugs, spheres and wire. Safe. | garnierite in Russia, South Africa, USA; pentlandite in Canada, South Africa | | 0.3 - 0.5 mg | 350 mg kg-1 in rat for nickel acetate | 80000 years | garnierite, millerite, nickeline, pentlandite, nickel-iron meteorites | 1726 K | 1.03 × 10-12 cm | 633 K |  | 115 pm | | 3F4 in ground state | a = 352.38 pm | 0.312 GPa | 15 mg for a 70 kg average person | 70 × 106 tonnes | II | 199.5 GPa | | | 23.359 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 177.3 GPa | | 6.59 cm3 | corrosion resistant, silvery-white, lustrous, malleable and ductile metal | 3005 K | nickel carbonyl is extremely toxic | essential to some species, and can act to stimulate metabolism | 62 pm for Ni3+ | 6.84 × 10-8 Ω m at 293 K | 58.6934 in units of 12C = 12.000 | garnierite, pentlandite | 156 kJ mol-1 from Ni to Ni- | 7440-02-0 for Chemical Abstracts System database | 7780 kg m-3 for liquid at 1726 K boiling point | 125 pm | 28 | ferromagnetic | | Stockholm, Sweden | 14 including nuclear isomers | 1 - 3 mg kg-1 | 5.7 × 10-4 p.p.m. in deep Pacific seawater | 361.939 nm for Ni I | acids by disolving except for concentrated HNO3 | nickel 58 | | Ni | alloys, especially stainless steel, coins, metal plating and catalysts | 510000 tonnes year-1 | [Ar]3d84s2 in ground state | 28 | 46.6 cm2 g-1 for MoKα X-ray diffraction | 1 - 2 p.p.m. in muscle | 1.91 Pauling | | | a column number in the table of the elements |
| nitrogen | niy-troh-jen | 1.91 barns | | pnictogen | 12 to 18 | 5.577 kJ mol-1 | D. Rutherford | 0.02598 W m-1 K-1 at 300 K for gas | 1772 | atmosphere of a planet or asteroid | nitron genes = nitre forming (potassium nitrate) from Greek | | 0.720 kJ mol-1 | small pressurized canisters. Safe. | | 3394 kPa | high | | 6000 years | nitratine, nitrammite, nitrobarite, nitrocalcite and nitromagnesite | 63.29 K | 0.936 × 10-12 cm | |  | 70 pm for single bond | | 4S3/2 in ground state | a = pm | | 1.8 kg for a 70 kg average person | × 10 tonnes | V | | | gas at standard temperature and pressure | 20.786 J K-1 mol-1 for atomic gas at constant pressure 0.1 MPa at 298.15 K | | 154 pm | 12.65 cm3 | colourless, odourless gas (N2) | 77.4 K | harmless gas, but it could asphyxiate if it excluded oxygen from the lungs | constituent element of DNA and amino acids; nitrogen cycle in nature | | | 14.00674 in units of 12C = 12.000 | liquified air | -7 kJ mol-1 from N to N- | 7727-37-9 for Chemical Abstracts System database | 1.2506 kg m-3 for gas at 273 K | 71 pm | 7 | -5.4 × 10-9 kg-1 m3 for gas | | Edinburgh, Scotland, UK | 8 including nuclear isomers | non-toxic as N2 gas but NO2, HCN and NH3 are toxic | p.p.m. in seawater | 1246.962 nm for N I | generaly unreactive at normal temperatures | nitrogen 14 | | N | fertilizers, acids (HNO3), explosives, plastics and dyes | 44 × 106 tonnes year-1 | [He]2s22p3 in ground state | 7 | 0.916 cm2 g-1 for MoKα X-ray diffraction | 72000 p.p.m. in muscle | 3.04 Pauling | 126.05 K | | 15 |
| palladium | | | | atom | | | | | | ocean | | | | | | | | | 50000 years | | | | |  | | | | | | | | II | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
| phosphorus | fos-for-us | 0.172 barns | | pnictogen | 26 to 36 | 51.9 kJ mol-1 for P4 solid | Hennig Brandt | 12.1 W m-1 K-1 for black phosphorus solid at 300 K | 1669 | in planet metallic core and associated with iron | phosphoros = bringer of light from Greek | 124.5 × 10-6 K-1 for P4 solid | 2.51 kJ mol-1 for P4 solid | white sticks (Danger!), red lumps or powder (Care!) | Russia, USA, Morocco, Tunisia, Togo, Nauru | | 900 - 1900 mg | 100 mg for white phosphorus in humans | 100000 years | apatite, phosphophyllite, turquoise, vivianite | 683 K for red phosphorus solid under pressure | 0.513 × 10-12 cm | |  | 110 pm for single bond | | 4S3/2 in ground state | a = 1131 pm for red phosphorus | | 780 g for a 70 kg average person | 5.7 × 109 tonnes | V | | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 190 pm | 17.02 cm3 | soft and flammable white solid, the red form is usually non-flammable | 553 K for P4 | white phosphorus chronic poisoning leads to necrosis of the jaw (phossy-jaw) | constituent of DNA, ATP and many other biochemical molecules. Phosphate cycle. | 212 pm for P3- | 1 × 109 Ω m for P4 solid at 293 K | 30.973762 in units of 12C = 12.000 | apatite, turquoise (ornamental stone) | 44 kJ mol-1 from to - | 7723-14-0 for Chemical Abstracts System database | 2690 kg m-3 for black phosphorus solid at 293 K | 115 pm for red form | 15 | -8.4 × 10-9 kg-1 m3 for red phosphorus solid | | Hamburg, Germany | 10 including nuclear isomers | 11 μg kg-1 for white phosphorus in rat | 0.084 p.p.m. in deep Pacific seawater | 1648.292 nm for P I | alkalis to form phosphine gas | phosphorus 31 | | P | fertilizers, insecticides, metal treatment, detergents and foods | 153 × 106 tonnes year-1 | [Ne]3s23p3 in ground state | 15 | 7.89 cm2 g-1 for MoKalpha X-ray diffraction | 3000 - 8500 p.p.m. in muscle | 2.19 Pauling | 994 K | | 15 |
| samarium | | | | lanthanide | | | | | | ocean | | | | | | | | | 200 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| scandium | | | | atom | | | | | | ocean | | | | | | | | | 5000 years | | | | |  | | | | | | | | III] | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
| selenium | | | | chalcogen | | | | | | in sulfide minerals | | | | | | | | | 3000 years | | | | |  | | | | | | | | VI some IV | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 16 |
| silicon | sil-i-kon | 0.171 barns | 39.7 GPa | atom | 24 to 34 | 383.3 kJ mol-1 | J.J. Berzelius | 148 W m-1 K-1 at 300 K | 1824 | in silicate materials such as igneous rocks | silicis = flint from Latin | 4.2 × 10-6 K-1 | 39.6 kJ mol-1 | available as powder, pieces or lumps. Safe. | talc in Austria, Italy, India, South Africa, Australia, mica in Canada, USA, India, Brazil | | 18 - 1200 mg | non-toxic | 30000 years | silicates | 1683 K | 0.41543 × 10-12 cm | |  | 117 pm | | 3P0 in ground state | a = pm | 0.42 GPa | 1 g for a 70 kg average person | unlimited × 10 tonnes | IV | 113 GPa | HF acid or hot alkali solutions by dissolving | | 22.251 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 200 pm | 12.06 cm3 | ultrapure crystals of silicon have a blue-grey metallic sheen | 2628 K | some silicate fibres are carcinogenic such as asbestos | essential to some species and possibly to humans | 271 pm for Si4- | 0.001 Ω m at 273 K | 28.0555 in units of 12C = 12.000 | quartz, talc, mica | 133.3 kJ mol-1 from Si to Si- | 7440-21-3 for Chemical Abstracts System database | 2525 kg m-3 for liquid at 1683 K melting point | 117 pm | 14 | -1.8 × 10-9 kg-1 m3 for solid | | Stockholm, Sweden | 11 including nuclear isomers | non-toxic | 4.09 p.p.m. in deep Pacific seawater | 637.136 nm for Si II | | silicon 28 | | Si | semiconductors, alloys and polymers | 3.4 × 106 tonnes year-1 for metallurgical grade | [Ne]3s23p2 in ground state | 14 | 6.44 cm2 g-1 for MoKα X-ray diffraction | 100 - 200 p.p.m. in muscle | 1.90 Pauling | | | 14 |
| silver | | | | atom | | | | | | in sulfide minerals | | | | | | | | | 5000 years | | | | |  | | | | | | | | I | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
| strontium | | | | atom | | | | | | ocean | | | | | | | | | 4 × 106 years | | | | |  | | | | | | | | II | | | | | | | | | | | | | | | | | | | | | | malleable, extrudable and machinable | | | | | | | | | | | | | | | | | | | |
| terbium | | | | lanthanide | | | | | | ocean | | | | | | | | | 103 to 105 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| thulium | | | | lanthanide | | | | | | ocean | | | | | | | | | 103 to 105 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| vanadium | van-ay-di-uhm | 5.08 barns | 46.7 GPa | atom | 44 to 55 | 458.6 kJ mol-1 | A.M. del Rio | 30.7 W m-1 K-1 at 300 K | 1801 | ocean | Vanadis = Scandinavian goddess from Scandinavian | 8.3 × 10-6 K-1 | 17.6 kJ mol-1 | foil, granules, powder, rod, turnings. Care ! | not mined as such, obtained as ore by-product and from Venezuelan oils | | 0.04 mg | 10 mg kg-1 V2O5 ingested by rat | 50000 years | carnotite, descloizite, patronite, vanadinite | 2160 K | -0.0382 × 10-12 cm | |  | | | 4F3/2 in ground state | a = 302.40 pm | 0.365 GPa | 0.11 mg for a 70 kg average person | | V | 127.6 GPa | | | 26.012 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 158 GPa | | 8.34 cm3 | shiny, silvery metal, soft when pure. Resists corrosion due to a protective film of oxide | 3650 K | mutagenic effect in some experimental compounds | essential to some species including humans; stimulates metabolism | 72 pm for V2+ | 24.8 × 10-8 Ω m at 293 K | 50.9415 in units of 12C = 12.000 | descloizite, patronite, vanadinite, carnotite | 50.7 kJ mol-1 from V to V- | 7440-62-2 for Chemical Abstracts System database | 5550 kg m-3 for liquid at 2160 K melting point | 132 pm | | +6.28 × 10-8 kg-1 m3 for solid | | Mexico City, Mexico | 11 including nuclear isomers | varies | 1.8 × 10-3 p.p.m. in deep Pacific seawater | 399.864 nm for V I | concentrated acids | vanadium 51 | | V | alloys, especially in steel | 7000 tonnes year-1 | [Ar]3d34s2 in ground state | 23 | 27.5 cm2 g-1 for MoKalpha X-ray diffraction | 0.02 p.p.m. in muscle | 1.63 Pauling | | | a column number in the table of the elements |
| ytterbium | | | | lanthanide | | | | | | ocean | | | | | | | | | 400 years | | | | |  | | | | | | | | III | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 4f | | | | | | | | | | | a column number in the table of the elements |
| zinc | | | | atom | | | | | | in sulfide minerals | | | | | | | | | 5000 years | | | | |  | | | | | | | | II | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
 Astronomy View all facts Glossary Help
Astronomy View all facts Glossary Help Astronomy View all facts Glossary Help
Astronomy View all facts Glossary Help

































 Next oceanic element: scavenged oceanic element Up: oceanic element Previous oceanic element: accumulating oceanic element
Next oceanic element: scavenged oceanic element Up: oceanic element Previous oceanic element: accumulating oceanic element