 Astronomy View all facts Glossary Help
Astronomy View all facts Glossary Help Astronomy View all facts Glossary Help Astronomy View all facts Glossary Help |
| physical object > natural object > particle > element > metallic element > alkali earth metal > calcium |
| calcium (Ca, 1S0 in ground state) | ||||
| subject | fact | |||
| calcium | has symbol Ca |  |
| has lethal intake 6.45 grams kg-1 in carbonate form for rat |  | |
| has reserves almost unlimited |  | |
| has main mining area common everywhere |  | |
| has mineral anhydrite, aragonite, calcite, dolomite, gypsum, shortite, vaterite |  | |
| has neutron scattering length 0.476 × 10-12 cm |  | |
| has electron affinity -186 kJ mol-1 from Ca to Ca- |  | |
| has toxic intake non-toxic |  | |
| has poisson's ratio 0.31 GPa |  | |
| has bulk modulus 17.2 GPa |  | |
| has rigidity modulus 7.9 GPa |  | |
| has young's modulus 19.6 GPa |  | |
| has mass magnetic susceptibility +1.4 × 10-8 kg-1 m3 for solid |  | |
| has electrical resistivity 3.43 × 10-8 Ω m at 293 K |  | |
| has covalent radii 174 pm |  | |
| has thermal neutron capture cross section 0.43 barns |  | |
| has longest lived isotope |  | |
| has isotope mass range 36 to 51 |  | |
| has number of isotopes 16 including nuclear isomers |  | |
| has pronunciation kal-sium |  | |
| has name origin calx = lime from Latin |  | |
| has discovery location London, England |  | |
| has abundance 2.24 × 106 in Sun relative to H = 1 × 1012 |  | |
| has abundance 41000 p.p.m. in Earth's crust |  | |
| has abundance 390 p.p.m. in Atlantic surface seawater |  | |
| has abundance 430 p.p.m. in deep Atlantic seawater |  | |
| has abundance 390 p.p.m. in Pacific surface seawater |  | |
| has abundance 440 p.p.m. in deep Pacific seawater |  | |
| has specimen granules, pieces or turings. Care ! |  | |
| has world production 2000 tonnes year-1 for calcium metal |  | |
| has world production 112 × 106tonnes year-1 for lime, CaO |  | |
| has chief source calcite, dolomite, gypsum (used in cement and plaster) anhydrite (used to make H2SO4) |  | |
| has mass absorption coefficient 162 cm2 g-1 for CuKα X-ray diffraction |  | |
| has mass absorption coefficient 18.3 cm2 g-1 for MoKα X-ray diffraction |  | |
| has space group |  | |
| has crystal cell dimension a = pm |  | |
| has crystal type |  | |
| has atomic emission line 239.856 nm for Ca I |  | |
| has atomic emission line 317.933 nm for Ca II |  | |
| has atomic emission line 373.690 nm for Ca II |  | |
| has atomic emission line 393.366 nm for Ca II (strong) |  | |
| has atomic emission line 393.847 nm for Ca II |  | |
| has atomic emission line 422.673 nm for Ca I (used in atom absorption spectrometry) |  | |
| has term symbol 1S0 in ground state |  | |
| has electron configuration [Ar]4s2 in ground state |  | |
| has mass of element in person 1.00 kg for a 70 kg average person |  | |
| has daily dietary intake 600 - 1400 mg |  | |
| has level in humans 60.5 mg dm-3 in blood |  | |
| has level in humans 170000 p.p.m. in bone |  | |
| has level in humans 100 - 360 p.p.m. in liver |  | |
| has level in humans 140 - 700 p.p.m. in muscle |  | |
| has hazard calcium compounds are only toxic via the other elements they contain |  | |
| has biological role essential to all species |  | |
| has linear expansion coefficient 22 × 10-6 K-1 |  | |
| has thermal conductivity 200 W m-1 K-1 at 300 K |  | |
| has molar volume 25.86 cm3 |  | |
| has heat capacity 25.31 J K-1 mol-1 for solid α form at constant pressure 0.1 MPa at 298.15 K |  | |
| has heat capacity 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K |  | |
| has heat of vaporization 149.95 kJ mol-1 |  | |
| has heat of fusion 9.33 kJ mol-1 |  | |
| has boiling point 1757 K |  | |
| has melting point 1112 K |  | |
| has electronegativity 1.00 Pauling |  | |
| has relative atomic mass 40.078 in units of 12C = 12.000 |  | |
| has ionic radii 106 pm for Ca2+ |  | |
| has atomic radii 197 pm for α-form |  | |
| has number of protons 20 |  | |
| has registry number 7440-70-2 for Chemical Abstracts System database |  | |
| has density 1550 kg m-3 for solid at 293 K |  | |
| has density 1365 kg m-3 for liquid at 1112 K melting point |  | |
| has synthesis mechanism heat calcium oxide (CaO) with aluminium metal in vacuum |  | |
| has ocean oxidation state II |  | |
| has ocean residence time 1 × 106 years |  | |
| has atomic number 20 |  | |
| reacts with oxygen and water but soon forms a thin protective oxide-nitride film preventing further corrosion |  | |
| has discovery date 1808 (isolated) |  | |
| has discoverer Sir Humphry Davy |  | |
| has uses alloys and in the manufacture of zirconium, thorium, and uranium and the rare earth metals |  | |
| has uses CaO used in metallurgy, water treatment, chemicals industry, cement, etc |  | |
has image 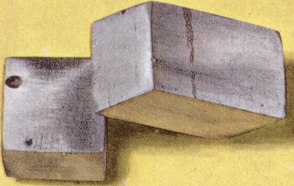 |  | |
| has definition silvery-white, relatively soft metal |  | |
| is a kind of alkali earth metal |  | |
| is a kind of lithophile element |  | |
| is a kind of recycled oceanic element |  | |
| alkali earth metal | has hardness malleable, extrudable and machinable |  |
| recycled oceanic element | has ocean concentration increasing with depth |  |
| lithophile element | has occurrence in silicate materials such as igneous rocks |  |
| element | is a part of Universe |  |
| has synonym atom |  | |
| particle | obeys uncertainty principle |  |
| has frequency inversely proportional to the wavelength |  | |
| has wavelength inversely proportional to its momentum |  | |
| has charge |  | |
| has mass |  | |
| physical object | has location or center of gravity |  |
| has angular momentum |  | |
| has velocity |  | |
| has momentum |  | |
| has temperature |  | |
| has volume |  | |
| has extent |  | |
| has material |  |
Kinds of calcium :
 Next alkali earth metal: magnesium Up: alkali earth metal, lithophile element, recycled oceanic element Previous alkali earth metal: beryllium
Next alkali earth metal: magnesium Up: alkali earth metal, lithophile element, recycled oceanic element Previous alkali earth metal: beryllium