| Subject |
has pronunciation |
has space group |
has stable isotope |
has thermal neutron capture cross section |
has rigidity modulu |
has synonym |
has isotope mass range |
stable isotope |
has heat of vaporization |
has discoverer |
has thermal conductivity |
has discovery date |
has occurrence |
has name origin |
has linear expansion coefficient |
has heat of fusion |
has specimen |
has main mining area |
has critical pressure |
has daily dietary intake |
has lethal intake |
has ocean residence time |
has mineral |
has melting point |
has neutron scattering length |
has image |
has covalent radii |
has symbol name origin |
has term symbol |
has crystal cell dimension |
has level in human liver |
has mass of element in person |
has poisson's ratio |
has appearancee |
has reserve |
has ocean oxidation state |
has young's modulu |
has symbol origin |
has state |
has heat capacity |
has bulk modulu |
has van der Waals radii |
has molar volume |
has definition |
has boiling point |
has hazard |
has biological role |
has ionic radii |
has electrical resistivity |
has relative atomic mass |
has chief source |
has electron affinity |
has registry number |
has density |
has atomic radii |
has atomic number |
has mass magnetic susceptibility |
has hardness |
has discovery location |
has level in human bone |
has number of isotope |
has toxic intake |
has abundance |
has atomic emission line |
reacts with |
has longest lived isotope |
has filling orbital |
has symbol |
has use |
has crystal type |
has level in human muscle |
has world production |
has electron configuration |
has number of proton |
has level in human blood |
has mass absorption coefficient |
has level in human |
has electronegativity |
has critical temperature |
has group |
|---|
| aluminium | al-oo-min-iuhm | Fm3m | | | 26.2 GPa | atom | 22 to 31 | aluminium 27 | 293.72 kJ mol-1 | Oersted | 237 W m-1 K-1 at 300 K | 1825 | ocean | alumen = alum from latin | 23.03 K-1 | 10.67 kJ mol-1 | foil, granules, ingots, pellets, powder, rod, shot or wire. Safe. Aluminum powder can react dangerously with other materials. | Surinam, Jamaica, Ghana, Indonesia, Russia | | 2.45 mg | | 150 years | bauxite, boehmite, diaspore, gibbsite, andalusite, corundum, sillimanite, topaz | 933.52 K | 0.3449 × 10-12 cm |  | 125 pm | | 2P1/2 in ground state | a = 404.959 pm | 3 - 23 p.p.m. | 60 mg for a 70 kg average person | 0.345 GPa | | 6 × 106 tonnes | III | 70.6 GPa | | | 21.38 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 75.2 GPa | 205 pm | 10.00 cm3 | soft and malleable metal | 2740 K | accumulates in the body from daily intake, compounds are used as food additives and in indigestion tablets | none | 57 pm for Al3+ | 2.6548 × 108 Ω m at 293 K | 26.981539 in units of 12C = 12.000 | bauxite | 44 kJ mol-1 from Al to Al- | 7429-90-5 for Chemical Abstracts System database | 2390 kg m-3 for liquid at 933.52 K melting point | 143 pm | 13 | 7.7 × 109 kg-1 m3 for solid | | Copenhagen, Denmark | 4 - 27 p.p.m. | 11 | 5 g | 0.13 × 10-4 p.p.m. in deep Pacific seawater | 396.152 nm for Al I (strong) | air to produce a thin protective oxide layer | aluminium 27 which is stable | | Al | vehicle, aircraft and construction industries | f.c.c. | 0.7 - 28 p.p.m. | 15 × 106 tonnes per year | [Ne]3s23p1 in ground state | 13 | 0.39 mg dm-3 | 5.16 cm2 g-1 for MoKα X-ray diffraction | | 1.61 Pauling | | 13 |
| antimony | anti-moni | P63/mmc for metal form | antimony 123 | 4.91 barns | 20.7 GPa | pnictogen | 108 to 136 | | 67.91 kJ mol-1 | | 24.3 W m-1 K-1 at 300 K | 1600 BC probably known to the ancients and certainly to the alchemists | ocean | anti + monos = not alone from greek | 8.5 × 10-6 K-1 | 20.9 kJ mol-1 | available as pieces, powder or shot. Care ! | China, Italy, Peru, Mexico, Bolivia, France | | 0.002 to 1.3 mg | 140 mg for antimony potassium tartrate (oral) for LD50 | 3.5 × 105 years | sibiconite, stibnite, tetrahedrite, ullmannite | 903.89 K | 0.557 × 10-12 cm |  | 141 pm | | 4S3/2 in ground state | c = 533 pm for metal form | | 2 mg for a 70 kg average person | 0.25 to 0.33 GPa | | 2.5 × 106 tonnes | III | 54.7 GPa | stibium from latin | | 20.79 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 220 pm | 18.20 cm3 | metalloid element with three forms. The metallic form is the more stable and is bright, silvery, hard and brittle | 1908 K | small doses stimulate metabolism, large doses cause liver damage | none | 245 pm for Sb2- | 39.0 × 10-8 Ω m at 273 K | 112.760 in units of 12C = 12.000 | stibnite, tetrahedrite although mainly a copper ore yields antimony as a by-product | 101 kJ mol-1 from Sb to Sb- | 7440-36-0 for Chemical Abstracts System database | 6483 kg m-3 for liquid at 903.89 K melting point | 182 pm | 51 | -1.0 × 10-8 kg-1 m3 for solid | | | | 40 | 100 mg | 3 × 10-4 p.p.m. in seawater | 259.805 nm for Sb I | stable in dry air, not attacked by dilute acids or alkalis | | | Sb | hardenning other metals, stotage batteries, bearings | h.c.p. for metal form | | 53000 tonnes per year | [Kr]4d105s25p3 in ground state | 51 | | 33.1 cm2 g-1 for MoKα X-ray diffraction | 0.042 to 0.191 p.p.m. in muscle | 2.05 Pauling | | 15 |
| boron | bohr-on | R3m for α-B | | 767 barns | | atom | 8 to 13 | | 538.9 kJ mol-1 | L.J. Lussac and L.J. Thenard | 27.0 W m-1 K-1 at 300 K | 1808 | ocean | buraq from Arabic | 5 × 10-6 K-1 | 22.2 kJ mol-1 | crystals, pieces or powder. Safe. | ulexite in USA, Tibet, Chile; colemanite in USA, Turkey | | 1 - 3 mg | 10 - 20 g as boric acid | 1 × 107 years | borax, colemanite, datolite, kernite, ulexite | 2573 K | 0.535 × 10-12 cm |  | 88 pm | | 2P1/2 in ground state | a = 506.7, α = 58deg4' pm for α-B | | 18 mg for a 70 kg average person | | | 270 × 106 tonnes as B2O3 | III | | | | 20.799 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 208 pm | 4.62 cm3 | Non-metal with several forms - the most common form is a dark amorphous powder, unreactive to water, acids and alkalis. Rare and fragile element. Nuclear reactions in stars destroy it. Most boron is created in space, by cosmic rays that smash into heavier atoms and split them. | 3931 K | boric acid and borates are poisonous, although once used in medicines | essential to plants; toxic in excess | 23 pm for B3+ | 18000 Ω m at 293 K | 10.811 in units of 12C = 12.000 | kernite, borax, ulexite, colemanite | 26.7 kJ mol-1 from B to B- | 7440-42-8 for Chemical Abstracts System database | 2340 kg m-3 for β-rhombohedral solid at 293 K | 83 pm | 5 | -7.8 × 10-9 kg-1 m3 for solid | | Paris, France and London, England | | 6 including nuclear isomers | 5 g as boric acid | 4.41 p.p.m. in seawater | 1166.247 nm for B I | metals to form borides | boron 11 | | B | borosilicate glass, detergents and fire-retardants | rhombohedral for α-B | | 1 × 106 tonnes year-1 as B2O3 | [He]2s22p1 in ground state | 5 | | 0.392 cm2 g-1 for MoKalpha X-ray diffraction | 0.33 - 1 p.p.m. in muscle | 2.04 Pauling | | 13 |
| bromine | | | | | | group VII element | | | | | | | atmosphere of a planet or asteroid | | | | | | | | | 1 × 108 years | | | |  | | | | | | | | | | -I | | | liquid at standard temperature and pressure | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 17 |
| cesium | | | | | | group I element | | | | | | | ocean | | | | | | | | | 600000 years | | | | 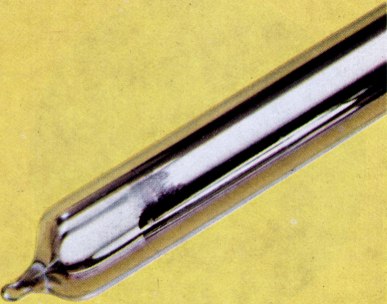 | | | | | | | | silvery except for francium | | I | | | | | | | | | | | | | | | | | | | | | | soft | | | | | | | water vigorously | | | | | | | | | | | | | | | 1 |
| chlorine | klor-een | P4/ncm | | 35.5 barns | | group VII element | 31 to 41 | | 20.4033 kJ mol-1 | C.W. Scheele | 0.0089 W m-1 K-1 for gas at 300 K | 1774 | in silicate materials such as igneous rocks | chloros = pale green from Greek | | 6.41 kJ mol-1 | Cl2 in small pressurized canisters. Danger! | vast deposits in USA, Poland, Russia, Germany, China, India, Australia | 7700 kPa | 3.00 - 6.50 g | Cl2 inhalation 500 p.p.m. for 5 minutes for humans | 4 × 108 years | halite, carnallite, sylvite | 172.17 K | 0.95770 × 10-12 cm |  | 99 pm | | 2P3/2 in ground state | a = 856, c = 612 pm | | 95 g for a 70 kg average person | | | > 1 × 1013 tonnes | -I | | | gas at standard temperature and pressure | 21.840 J K-1 mol-1 for atomic gas at constant pressure 0.1 MPa at 298.15 K | | 181 pm | 17.46 cm3 for solid at 113 K | yellow-green, dense, sharp-smelling gas (Cl2) which is a key industrial chemical | 239.18 K | Cl2 50 p.p.m. is dangerous even in short doses | chloride, Cl-, is essential to many species, including humans | 181 pm for Cl- | | 35.4527 in units of 12C = 12.000 | halite (rock salt) | 349.0 kJ mol-1 from Cl to Cl- | 7782-50-5 for Chemical Abstracts System database | 3.214 kg m-3 for gas at 273 K | | 17 | -7.2 × 10-9 kg-1 m3 for gas | | Uppsala, Sweden | | 13 including nuclear isomers | Cl2 is very toxic affecting the eyes and lungs at 3 p.p.m. in air; chloride is non-toxic | 18000 p.p.m. in seawater | 858.597 nm for Cl I | | chlorine 35 | | Cl | PVC | tetragonal | | 168 × 106 | [Ne]3s23p5 in ground state | 17 | | 11.4 cm2 g-1 for MoKα X-ray diffraction | 2000 - 5200 p.p.m. chloride in muscle | 3.16 Pauling | 417 K | 17 |
| fluorine | floor-een | Pm3n for β-F2 | | 0.0096 barns | | group VII element | 17 to 23 | | 6.548 kJ mol-1 | H. Moissan | 0.0279 W m-1 K-1 at 300 K | 1886 (isolated) | ocean | fluere = to flow from Latin | | 5.10 kJ mol-1 | not available for sale as pure gas because it is too reactive and dangerous | Canada, USA, UK, Russia, Mexico, Italy | 5573 kPa | 0.3 - 0.5 mg | 5 - 25 g NaF | 400000 years | apatite, cryolite, fluorite | 53.53 K | 0.5654 × 10-12 cm |  | 58 pm | | 2P3/2 in ground state | a = 667 pm for β-F2 | | 2.6 g for a 70 kg average person | | | 123 × 106 tonnes | -I | | | gas at standard temperature and pressure | 22.744 J K-1 mol-1 for atomic gas at constant pressure 0.1 MPa at 298.15 K | | 135 pm | 18.05 cm3 | pale yellow gas (F2) which is the most reactive of all the elements, and is the strongest oxidizing agent | 85.01 K | organic fluorides are often quite harmless | essential in trace quantities for mammals, including humans, in the form of fluoride (F-) | 133 pm for F- | | 18.9984032 in units of 12C = 12.000 | fluorite | 328 kJ mol-1 from F to F- | 7782-41-4 for Chemical Abstracts System database | 1.696 kg m-3 for gas at 273 K | 70.9 pm | 9 | | | Paris, France | | 7 including nuclear isomers | 250 mg NaF | 0.4 × 10-4 p.p.m. in deep Pacific seawater | 712.789 nm for F I | almost everything violently | fluorine 19 | | F | AlF3 in aluminium production | cubic for β-F2 | | 4.7 × 106 tonnes year-1 for fluorite (CaF2) | [He]2s22p5 in ground state | 9 | | 1.80 cm2 g-1 for MoKalpha X-ray diffraction | 0.05 p.p.m. in muscle | 3.98 Pauling | 144.3 K | 17 |
| lithium | lith-iuhm | Fm3m for β-Li | lithium 6, lithium 7 | 70.5 barns | 4.24 GPa | group I element | 5 to 9 | | 134.7 kJ mol-1 | J.A. Arfvedson | 84.7 W m-1 K-1 at 300 K | 1817 | ocean | lithos = stone from Greek | 56 × 10-6 K-1 | 4.60 kJ mol-1 | chunks, ingot, powder, ribbon, rod, shot or wire. Care ! | USA, brines of Searles Lake, California | | 0.1 - 2 mg | 525 mg kg-1 carbonate ingested by rat | 2 × 106 years | amblygonite, lepidolite, petalite, spodumene | 453.69 K | -0.190 × 10-12 cm |  | 123 pm | | 2S1/2 in ground state | a = 437.9 pm for β-Li | | 7 mg for a 70 kg average person | 0.36 GPa | silvery except for francium | 7.3 × 106 tonnes | I | 4.91 GPa | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | | 13.00 cm3 | Soft, silvery-white, metal. Lightest of all solid elements, third in the periodic table after hydrogen and helium. Its atom comprises one proton and three electrons. One of the electrons is at a higher energy level than the other two. Some lithium formed in the big bang, along with huge amounts of hydrogen and helium. | 1620 K | moderately toxic by ingestion but there are wide variations of tolerances. | none; but lithium acts to stimulate metabolism and can control manic-depressive disorders | 78 pm for Li+ | 8.55 × 10-8 Ω m at 273 K | 6.941 in units of 12C = 12.000 | petalite, lepidolite | 59.6 kJ mol-1 from Li to Li- | 7439-93-2 for Chemical Abstracts System database | 515 kg m-3 for liquid at 453.69 K melting point | 152 pm | 3 | +2.56 × 10-8 kg-1 m3 for solid | soft | Stockholm, Sweden | | 5 including nuclear isomers | 20 - 200 g | 0.17 p.p.m. in seawater | 670.791 nm for Li I (strong, used in atom absorption spectrometry) | oxygen and water slowly | lithium 7 | | Li | light-weight alloys, especially with aluminium and magnesium, greases, batteries, glass, medicine and nuclear bombs | f.c.c. for β-Li | | 39000 tonnes year-1 | [He]2s1 in ground state | 3 | | 0.217 cm2 g-1 for MoKalpha X-ray diffraction | 0.023 p.p.m. in muscle | 0.98 Pauling | | 1 |
| magnesium | mag-neez-iuhm | P63/mmc | | 0.063 barns | 17.3 GPa | atom | 20 to 31 | | 128.7 kJ mol-1 | Joseph Black | 156 W m-1 K-1 at 300 K | 1755 | in silicate materials such as igneous rocks | Magnesia = district of Thessaly from Greek | 26.1 × 10-6 K-1 | 9.04 kJ mol-1 | chips, granules, powder, ribbon, rod or turnings. Safe. | Austria, China, Poland, Russia, USA, India, Greece, Canada | | 250 - 380 mg | 8100 mg kg-1 for chloride, oral, rat | 1 × 107 years | brucite, carnalite, cordierite, diopside, dolomite, enstatite, epsomite, kiersite, magnesite, pyrope, spinel | 922.0 K | 0.5375 × 10-12 cm |  | 136 pm | | 1S0 in ground state | a = 320.94; c = 521.03 pm | | 19 g for a 70 kg average person | 0.291 GPa | | > 2 × 1010 tonnes as ores; and > 1 × 1024 tonnes in the sea | II | 44.7 GPa | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | 35.6 GPa | | 13.98 cm3 | silvery white, lustrous and relatively soft metal | 1363 K | compounds vary in toxicity but no evidence that metal produces systemic poisoning | essential to all species | 79 pm for Mg2+ | 4.38 × 10-8 Ω m at 293 K | 24.3050 in units of 12C = 12.000 | seawater; and the ores or dolomite, magnesite; carnallite and brucite | -21 kJ mol-1 from Mg to Mg- | 7439-95-4 for Chemical Abstracts System database | 1585 kg m-3 for liquid at 922.0 K melting point | 160 pm | 12 | +6.8 × 10-9 kg-1 m3 for solid | malleable, extrudable and machinable | Edinburgh, Scotland | | 12 including nuclear isomers | low toxicity | 1200 p.p.m. in seawater | 518.361 nm for Mg I | hot water | magnesium 24 | | Mg | as a 'sacrificial' electrode to protect other metals exposed to seawater and ground | h.c.p. | | 325000 tonnes year-1 | [Ne]3s2 in ground state | 12 | | 4.11 cm2 g-1 for MoKα X-ray diffraction | 900 p.p.m. in muscle | 1.31 Pauling | | |
| molybdenum | | | | | | atom | | | | | | | ocean | | | | | | | | | 600000 years | | | |  | | | | | | | | | | VI | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | a column number in the table of the elements |
| potassium | poh-tass-ium | Im3m | | 2.1 barns | 1.30 GPa | group I element | 35 to 51 | | 77.53 kJ mol-1 | Sir Humphry Davy | 102.4 W m-1 K-1 at 300 K | 1807 (isolated) | in silicate materials such as igneous rocks | potash from English | 83 × 10-6 K-1 | 2.40 kJ mol-1 | metal chunks (in mineral oil) or ingots. Warning! | Germany, Spain, Canada, USA, Italy | | 1.4 - 7.4 g | 2600 mg kg-1 | 21000 years | alunite, carnalite, orthoclase (mined for porcelain, ceramics and glass), sylvite | 336.80 K | 0.367 × 10-12 cm |  | 203 pm | kalium from Greek | 2S1/2 in ground state | a = 533.4 pm | | 140 g for a 70 kg average person | 0.35 GPa at 83 K | silvery except for francium | > 1 × 1010 tonnes | I | 3.53 GPa at 83 K | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 231 pm | 45.36 cm3 | soft white metal which is silvery when first cut but oxidizes rapidly in air | 1047 K | excess ingestion of KCl (dietary supplement) can be fatal | essential to all living things | 133 pm for K+ | 6.15 × 10-8 Ω m at 273 K | 39.0983 in units of 12C = 12.000 | sylvite, carnalite, alunite | 48.4 kJ mol-1 from K to K- | 7440-09-7 for Chemical Abstracts System database | 828 kg m-3 for liquid at 336.80 K melting point | 227 pm | 19 | +6.7 × 10-9 kg-1 m3 for solid | soft | London, England | | 18 including nuclear isomers | 4 g of KCl | 5 × 106 p.p.m. in seawater | 769.896 nm for K I | water violently | potassium 39 | | K | compounds are used in fertilizers, chemicals and glass | b.c.c | | 51 × 106 tonnes year-1 for salts | [Ar]4s1 in ground state | 19 | | 15.8 cm2 g-1 for MoKalpha X-ray diffraction | 16000 p.p.m. in muscle | 0.82 Pauling | | 1 |
| rubidium | | | | | | group I element | | | | | | | ocean | | | | | | | | | 800000 years | | | | 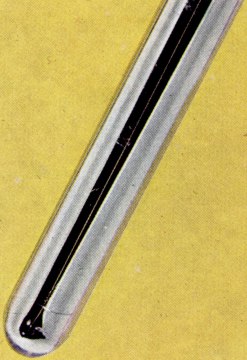 | | | | | | | | silvery except for francium | | I | | | | | | | | | | | | | | | | | | | | | | soft | | | | | | | water vigorously | | | | | | | | | | | | | | | 1 |
| sodium | so-dee-uhm | P63/mmc for α-Na | | 0.530 barns | 2.53 GPa | group I element | 19 to 31 | | 89.04 kJ mol-1 | Sir Humphry Davy | 141 W m-1 K-1 at 300 K | 1807 (isolated) | in silicate materials such as igneous rocks | soda from English | 70.6 × 10-6 K-1 | 2.64 kJ mol-1 | ingots or lumps, in sealed ampoules under nitrogen, or spheres and sticks stored under mineral oil. Warning! | halite in Germany, Poland, USA, UK; trona in Kenya, USA | | 2 - 15 g | 3000 mg kg-1 of chloride in rat | 1 × 108 years | halite, trona, occurs in many others but these are not mined as a source of sodium | 370.96 K | 0.358 × 10-12 cm |  | | natrium from Latin | 2S1/2 in ground state | a = 376.7, c = 615.4 pm for α-Na | | 100 g for a 70 kg average person | 0.34 GPa | silvery except for francium | almost unlimited | I | 6.80 GPa | | | 20.786 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 231 pm | 23.68 cm3 | soft, silvery-white metal which oxidizes rapidly when cut | 1156.1 K | compounds are not hazardous, but excess sodium chloride can be toxic by ingestion | essential to most species including humans | 98 pm for Na+ | 4.2 × 10-8 Ω m at 273 K | 22.989768 in units of 12C = 12.000 | halite, trona | 52.9 kJ mol-1 from to - | 7440-23-5 for Chemical Abstracts System database | 928 kg m-3 for liquid at 370.96 K melting point | 154 pm | 11 | +8.8 × 10-9 kg-1 m3 for solid | soft | Royal Institution, London, England | | 14 including nuclear isomers | 12 g kg-1 of chloride in humans | 10500 p.p.m. in seawater | 819.482 nm for Na I | water by producing hydrogen gas | sodium 23 | | Na | nuclear reactor heat exchanger | hexagonal for α-Na | | 200000 tonnes year-1 as sodium metal | [Ne]3s in ground state | 11 | | 3.21 cm2 g-1 for MoKalpha X-ray diffraction | 2600 - 7800 p.p.m. in muscle | 0.93 Pauling | | 1 |
| sulfur | sul-fer | Fddd for α form | | 0.53 barns | | chalcogen | 29 to 39 | | 9.62 kJ mol-1 | | 0.269 W m-1 K-1 for α at 300 K | prehistoric | in sulfide minerals | sulvere = sulfur from Sanskrit (sulphurium from Latin) | 74.33 × 10-6 K-1 | 1.23 kJ mol-1 | powder and flakes. Safe. | USA (native sulfur), Spain | 20700 kPa | 850 - 930 mg | 175 mg kg-1 for rabbits | 8 × 106 years | occurs naturally as native sulfur deposits associated with oil-bearing strata | 386.0 K for α form | 0.2847 × 10-12 cm |  | 104 pm | | 3P2 in ground state | a = 1046.46, b = 1286.60, c = 2448.60 pm for α form | | 140 g for a 70 kg average person | | | 2.5 × 109 tonnes | VI | | | | 23.673 J K-1 mol-1 for gas at constant pressure 0.1 MPa at 298.15 K | | 185 pm | 15.49 cm3 | the α-S8 orthorhombic form of sulfur is yellow | 717.824 K | elemental form is harmless unless ingested; ignited it emits highly toxic SO2 fumes | essential to all living things; part of the amino acids methionine and cysteine | 184 pm for S2- | 2 × 1015 Ω m at 293 K | 32.066 in units of 12C = 12.000 | native sulfur, pyrite, H2S in natural gas | 200.4 kJ mol-1 from S to S- | 7704-34-9 for Chemical Abstracts System database | 1819 kg m-3 for liquid at 393 K | 104 pm | | -5.83 × 10-9 kg-1 m3 for β solid | | | | 11 including nuclear isomers | elemental form is not very toxic, but simple derivatives are (SO2, H2S, etc.) | 870 p.p.m. in seawater | 964.99 nm for S I | oxidising acids | sulfur 32 | | S | key industrial chemical, starting point for sulfuric acid | orthorhombic for α form | | 54 × 106 | [Ne]3s23p4 in ground state | 16 | | 9.55 cm2 g-1 for MoKα X-ray diffraction | 5000 - 11000 p.p.m. in muscle | 2.58 Pauling | 1314 K | 16 |
| thallium | | | | | | atom | | | | | | | ocean | | | | | | | | | 10000 years | | | | 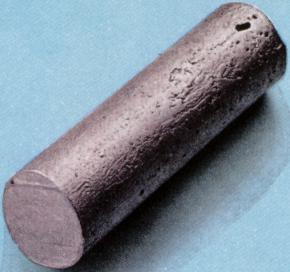 | | | | | | | | | | I | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 13 |
| uranium | | | | | | atom | | | | | | | ocean | | | | | | | | | 300000 years | | | |  | | | | | | | | | | VI | | | | | | | | | | | | | | | | | | | | 89 to 103 | | | | | | | | | | | 5f | | | | | | | | | | | | | a column number in the table of the elements |
 Astronomy View all facts Glossary Help
Astronomy View all facts Glossary Help Astronomy View all facts Glossary Help
Astronomy View all facts Glossary Help















 Next oceanic element: recycled oceanic element Up: oceanic element Previous oceanic element: unclassified oceanic element
Next oceanic element: recycled oceanic element Up: oceanic element Previous oceanic element: unclassified oceanic element